eDNA
PART 1
What is it?
How is it collected?



eDNA or Environmental DNA is a recently developed identification technique.
It uses an assortment of microscopic pieces of DNA to help tell the story of what creatures, plants, and other types of life has grown, swum, flown, or crawled through an environment.
“All critters leave stuff behind, little bits of scales and mucus and, if you're a crustacean, little bits of legs.
If you had recently swum through an area, there would even be little bits of you floating around!
eDNA is comprised of all those little bits of information.”


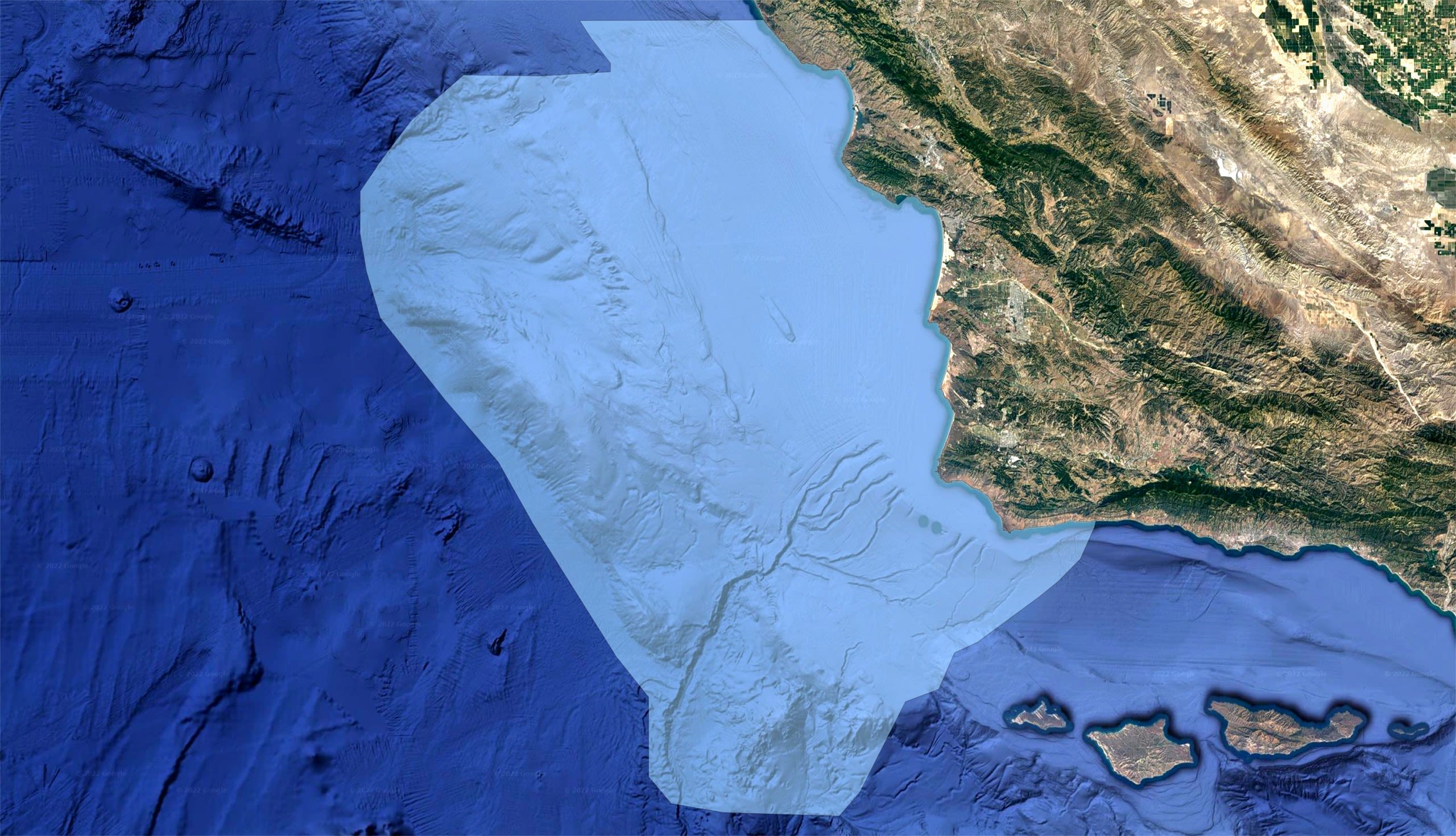
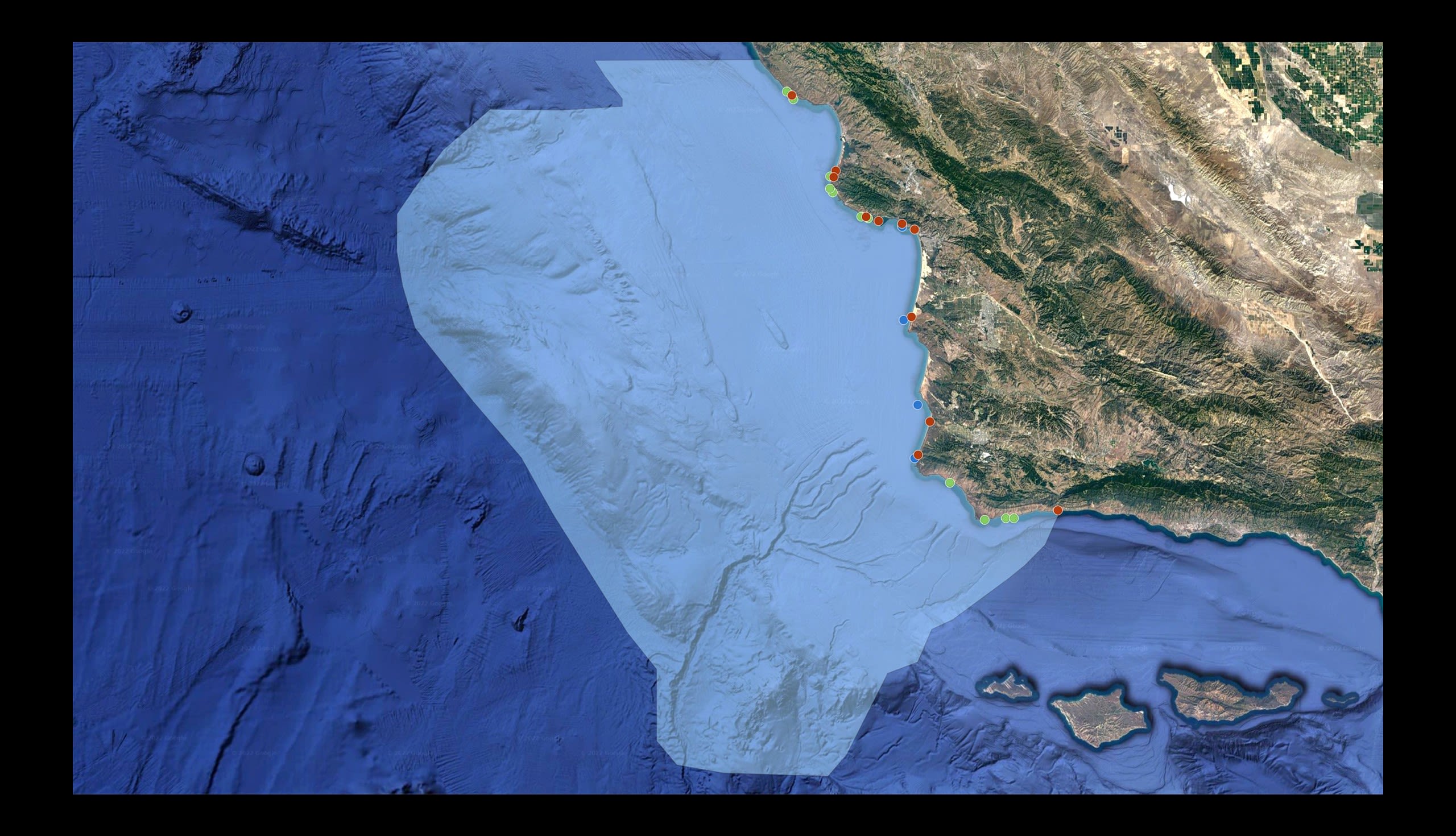
The proposed Chumash Heritage National Marine Sanctuary spreads across 7,000 square miles of ocean off the Central California Coast.
The Palumbi Lab at Stanford University has taken on the challenge to learn -
WHAT LIVES THERE?
Proposed Chumash Heritage
National Marine Sanctuary
Central CA Coast
Through various methods of collecting eDNA across the proposed marine sanctuary, Dr. Steve Palumbi and his team are creating a survey of life in those waters.




eDNA
•
HOW WE COLLECT IT
To begin the process, we determine our goals and strategize on how to accomplish them.
We determine the quantity, frequency, depths, and locations of the water and rock samples we need to collect. These will hold the DNA we are searching for.
Then we deploy a variety of techniques to gather the eDNA.

Kelp forest cobble sample, Palumbi Lab, Stanford University
Kelp forest cobble sample, Palumbi Lab, Stanford University

Preparing for underwater collection dive, Central CA Coast, Postdoc researchers, Palumbi Lab, Stanford University
Preparing for underwater collection dive, Central CA Coast, Postdoc researchers, Palumbi Lab, Stanford University

Brendan Cornwell, water sampling, Central CA Coast, Postdoc researcher, Palumbi Lab, Stanford University
Brendan Cornwell, water sampling, Central CA Coast, Postdoc researcher, Palumbi Lab, Stanford University

Steve Palumbi, hiking 10 miles to shore sampling site, Mussel Point, CA, Palumbi Lab, Stanford University
Steve Palumbi, hiking 10 miles to shore sampling site, Mussel Point, CA, Palumbi Lab, Stanford University
eDNA
•
Underwater Sampling
In the kelp forests, divers collect medium sized rocks that are covered with algae and other life. They bring the rocks to the surface for sampling. The geo-located samples provide fairly precise data as to when and where those plants and critters are located.
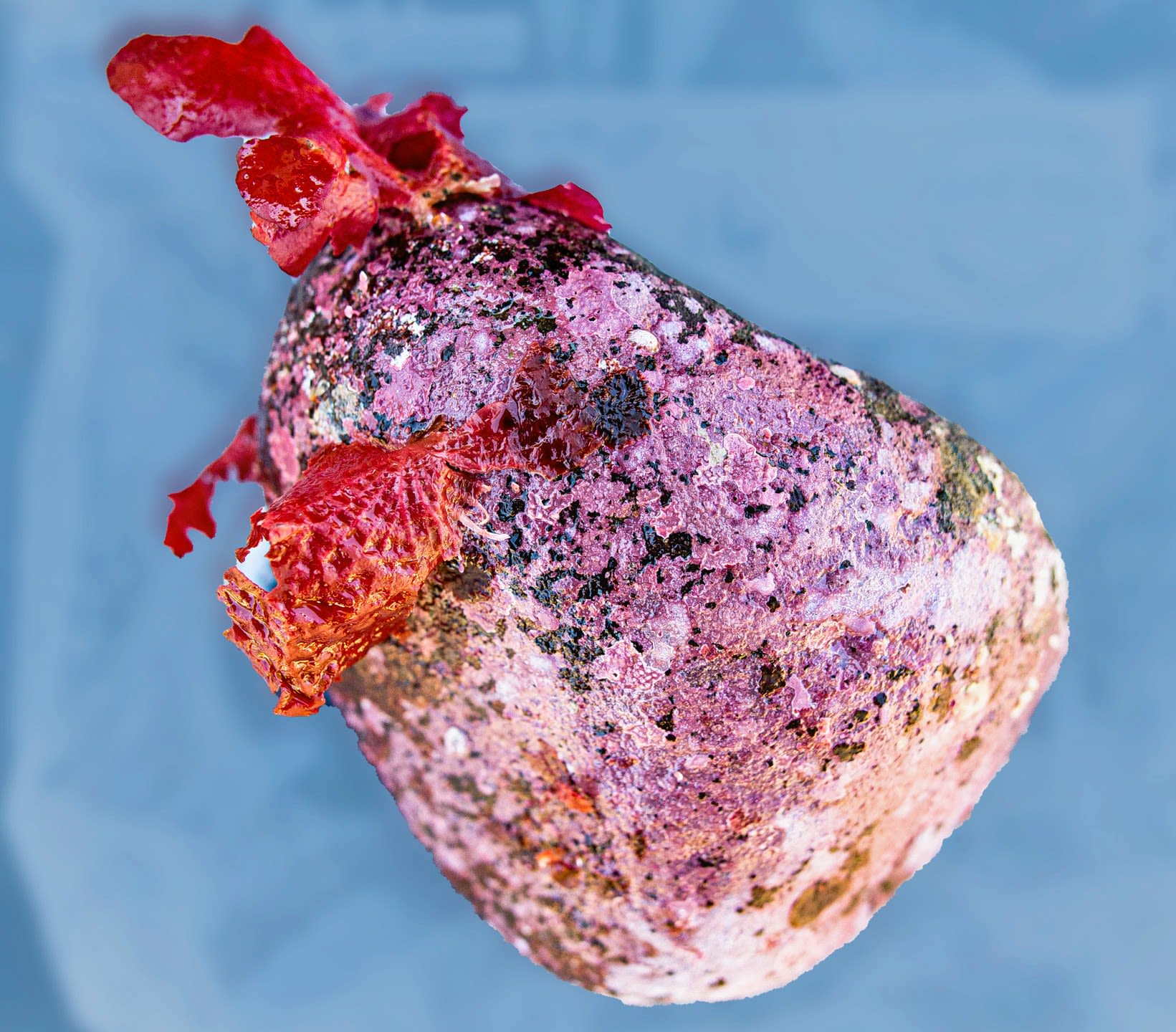
Kelp forest cobble sample, Palumbi Lab, Stanford University
Kelp forest cobble sample, Palumbi Lab, Stanford University
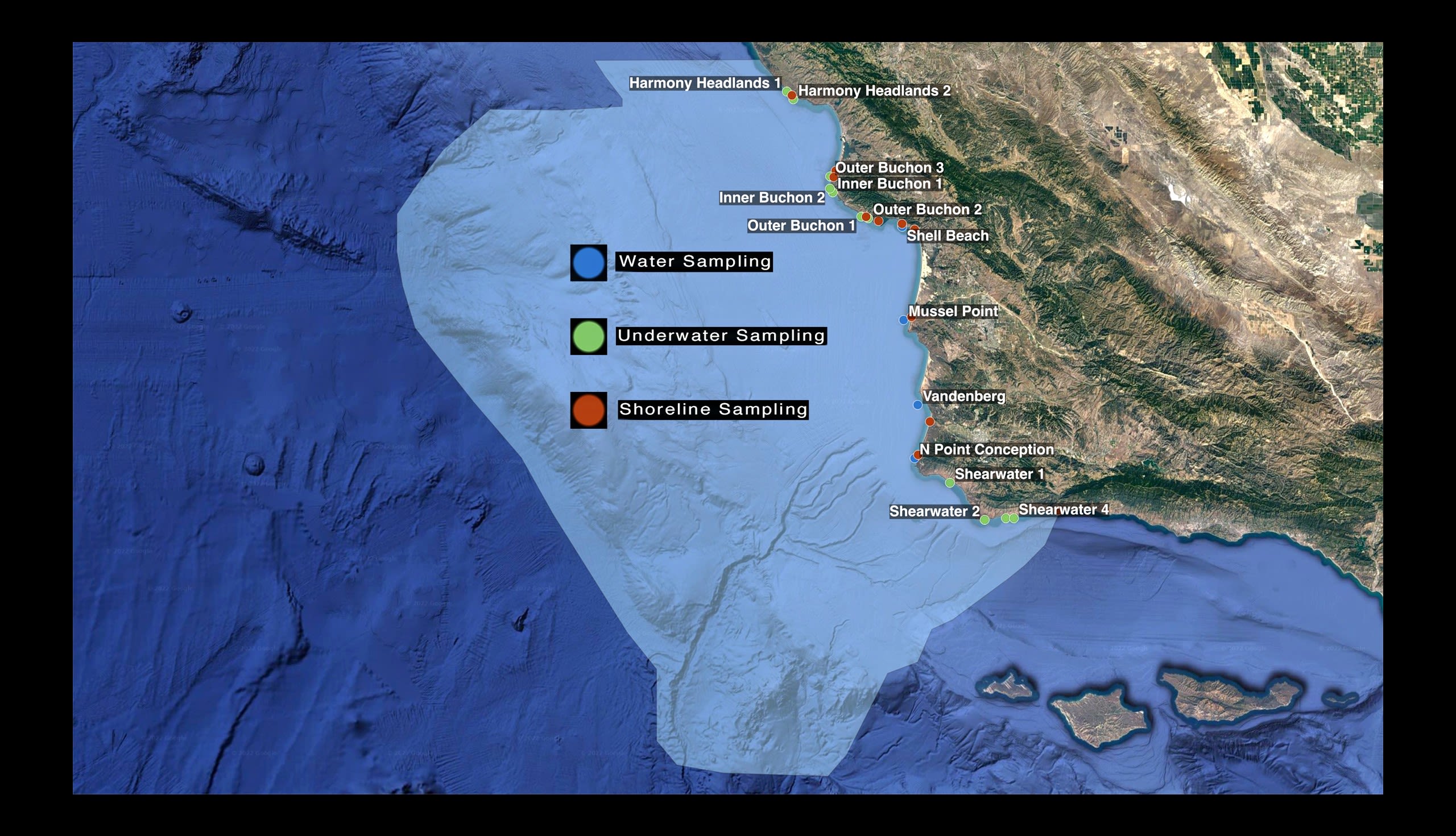
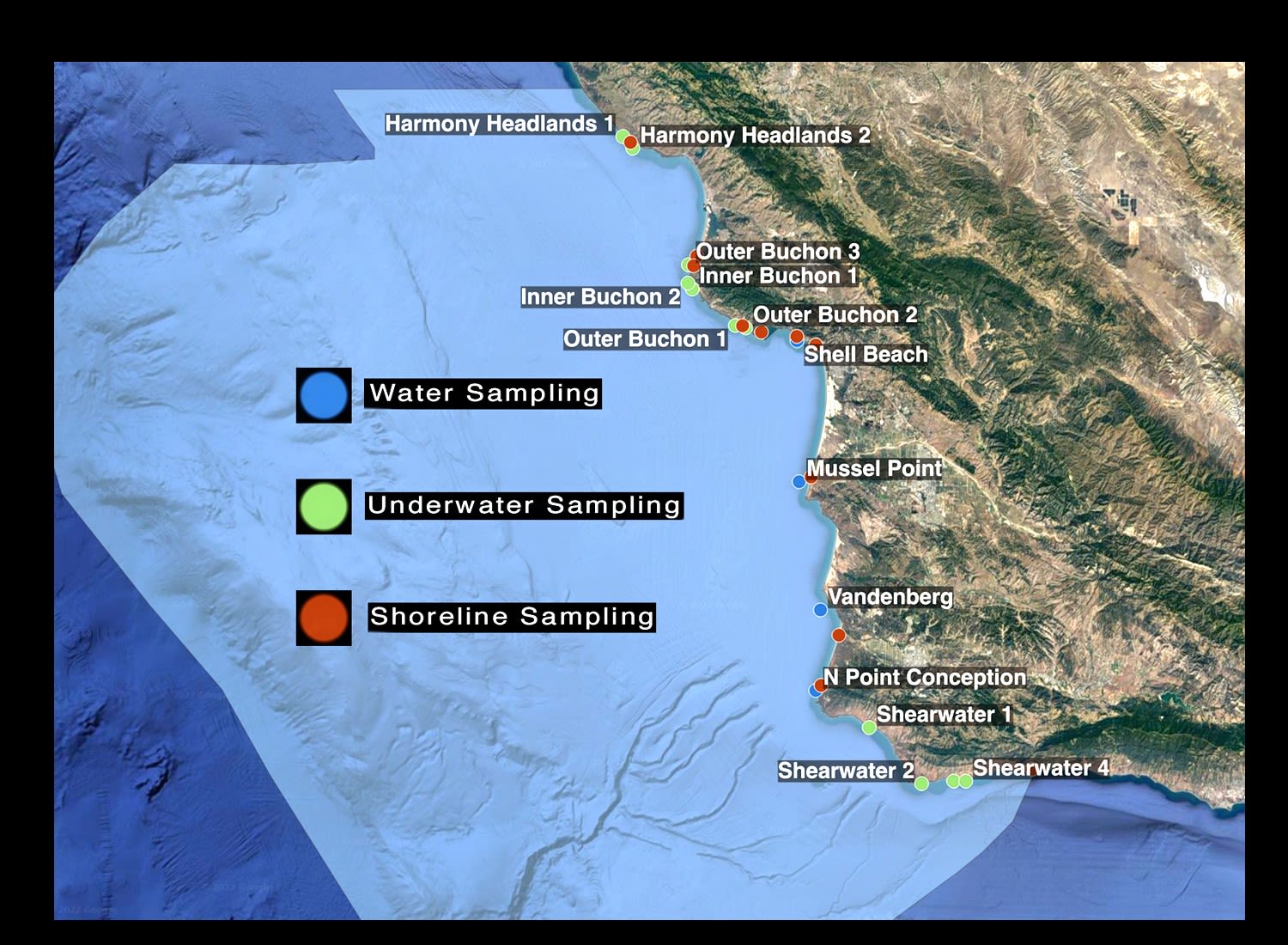
Underwater sampling occurs in about a dozen places along the coast;
scattered from the very northern boundary of the proposed marine sanctuary all the way down to the southern edge.

Marilla Lippert, underwater sampling, Central CA Coast, Lab Manager, Palumbi Lab, Stanford University
Marilla Lippert, underwater sampling, Central CA Coast, Lab Manager, Palumbi Lab, Stanford University

Kelp forest sample collection site, Central CA Coast, Palumbi Lab, Stanford University
Kelp forest sample collection site, Central CA Coast, Palumbi Lab, Stanford University

Dive Boat, heading out to collect underwater samples, Morro Harbor, CA, Postdoc researchers, Palumbi Lab, Stanford University
Dive Boat, heading out to collect underwater samples, Morro Harbor, CA, Postdoc researchers, Palumbi Lab, Stanford University

Dive Boat, preparing to collect underwater samples, Pismo Beach, CA, Postdoc researchers, Palumbi Lab, Stanford University
Dive Boat, preparing to collect underwater samples, Pismo Beach, CA, Postdoc researchers, Palumbi Lab, Stanford University
Underwater sampling process, Central CA Coast, Palumbi Lab, Stanford University
Underwater sampling process, Central CA Coast, Palumbi Lab, Stanford University
eDNA
•
Water Sampling
and Filtering
Water sampling is another important method to collect environmental DNA .
Water is collected in two ways: from a boat and from the shore.
From the boat, water is collected in bags, noting date, time and location.
The water is then squeezed out of the bagged samples, leaving the eDNA behind for future processing.
Brendan Cornwell and Marilla Lippert, boat water collection and filtering process, Central CA Coast, Palumbi Lab, Stanford University
Brendan Cornwell and Marilla Lippert, boat water collection and filtering process, Central CA Coast, Palumbi Lab, Stanford University

Steve Palumbi, collecting water samples from shore, Spooners Cove, CA, Palumbi Labs, Stanford University
Steve Palumbi, collecting water samples from shore, Spooners Cove, CA, Palumbi Labs, Stanford University

Steve Palumbi, collecting water samples from shore, Spooners Cove, CA, Palumbi Labs, Stanford University
Steve Palumbi, collecting water samples from shore, Spooners Cove, CA, Palumbi Labs, Stanford University

Processing shore water samples, Surf Beach, CA, Palumbi Labs, Stanford University
Processing shore water samples, Surf Beach, CA, Palumbi Labs, Stanford University

Processing shore water samples, Surf Beach, CA, Palumbi Labs, Stanford University
Processing shore water samples, Surf Beach, CA, Palumbi Labs, Stanford University
From the shore, water is similarly collected in bags, noting date, time and location.
After collecting the bagged water samples, the next step is to filter those samples down to the size of a bacteria. We use a filter that is two tenths of a micron. That equals two tenths of a millionth of a meter.
The holes in the filter are so small that it requires a lot of pressure (and time) to push the water through to catch the remaining eDNA. The team built a specialized filtering process to make it faster and easier.
The eDNA is caught in the filter and then ready for lab processing.
With the collection of the little piece of fish mucus floating in the water–there is no way to know if the fish was right there, 10 feet away, 100 feet away, or a half mile away. Maybe it was there within the last day or within the last several days.
From water-sampled eDNA, this method obtains a general understanding of what critters were in the vicinity from water-but nothing extremely specific.
eDNA
•
Shoreline
Sampling
eDNA is also collected along the shoreline. It is difficult for a boat to get to these places with the challenges of water depth, surf, cliffs , rocks and expense. Through the adaptation of the Metaprobe, an existing scientific tool, the team concocted another invention.
It is called the FRAMR - a Fishing Rod Actuated Metaprobe Retriever!
Using a fishing rod, a wiffle ball filled with a special type of collection gauze and a fishing weight is cast into the ocean.
As the wiffle ball sinks and bounces around in the water, the gauze collects eDNA.
Steve Palumbi, shoreline sample collecting, Harmony Head, CA, Palumbi Lab, Stanford University
Steve Palumbi, shoreline sample collecting, Harmony Head, CA, Palumbi Lab, Stanford University
With casting, it feels just like fishing, only it this case, it is fishing for eDNA.
And this simple method works!

Wiffle ball, Palumbi Labs, Stanford University
Wiffle ball, Palumbi Labs, Stanford University
At each location there are 10 casts per wiffle ball–any more casts and the wiffle ball tends to get lost. With each cast the wiffle ball bobs around for about a minute collecting eDNA and then it is cast again.
When finished, that particular wiffle ball and gauze is placed in a bag, noting the date, time and location. The sample is ready to be sent to the lab for processing.
From there it is time to walk down the shoreline to another location and start over.
We need to collect samples from
a variety of shoreline environments–
from kelp forests and the surf,
culverts and small channels,
near rocks and tide pools,
in both shallow and deep water–
all sorts of locations.
CURRENT SAMPLING LOCATIONS

eDNA
•
Calling all
Citizen Data Collectors

(from L to R) Bonnie Williamson, Joseph Lopez, Mia Lopez, and Steve Palumbi, walking to shore sampling site, Refugio State Beach, CA, Palumbi Labs, Stanford University
(from L to R) Bonnie Williamson, Joseph Lopez, Mia Lopez, and Steve Palumbi, walking to shore sampling site, Refugio State Beach, CA, Palumbi Labs, Stanford University

(from L to R) Bonnie Williamson, Joseph Lopez, Mia Lopez, and Steve Palumbi, walking to shore sampling site, Refugio State Beach, CA, Palumbi Labs, Stanford University
(from L to R) Bonnie Williamson, Joseph Lopez, Mia Lopez, and Steve Palumbi, walking to shore sampling site, Refugio State Beach, CA, Palumbi Labs, Stanford University
The shoreline collection process is very straightforward and opens the door for Citizen Data Collectors
to help us get samples.
We need thousands of data points to make this survey ever more thorough.
If you and your friends can provide the fishing poles–
the Palumbi Lab will designate local groups to send out a box of wiffle balls, gauze, fishing weights, collection bags, latex gloves and directions.
As a preservative, you might even add a splash of your own isopropyl alcohol into the collection bag to help stabilize the eDNA. (Even gin would work!)
Citizens can rig it all up, gather local eDNA and send those samples back for processing.
.
eDNA works well for the Chumash Heritage Marine Sanctuary and similar projects because it is a large scale approach; it scales up really well. In 10 minutes, individuals can collect a sample and get the resulting environmental DNA.
Using previous methods, a team might spend hours of scuba diving at a single location to get the same type of data.
A diver is great if samples are required from only one spot. But it becomes exponentially more difficult if samples are needed from 10, 100, or even 1,000 different locations.
In addition, collecting samples every month or six times a year, becomes increasingly expensive and time-consuming.
But with eDNA (and maybe your assistance) it all becomes possible!


COMING SOON!
How eDNA is processed and analyzed
©Steve Palumbi, Palumbi Labs, Stanford University
